Four steps to reduce cardiovascular disease
Cardiovascular disease is a policy choice
Cardiovascular disease (CVD) is the most common cause of death globally. In the US alone it kills about 2,000 people every day. Deaths from CVD include deaths from heart attacks, stroke, aneurysms, and cerebrovascular disease.1
We’ve already made great strides in lowering rates of CVD — in the 50 year period from 1950 to 1999 age-adjusted death rates from CVD fell by 56% according to the CDC.
The vast majority of CVD is caused by a very well-understood mechanism - atherosclerosis. While we have no good tools to reverse its progression,2 we do have several tools for preventing it. I won’t go into the mechanisms of atherosclerosis here, for that I recommend reading Outlive by Peter Attia.3
The landmark Framingham Heart Study showed that the absence of established modifiable CVD risk factors at an age of 50 years is associated with a 90% lower lifetime risk for men and a 79% lower risk for women.
So, it’s not crazy to claim that by getting people to stop smoking and implementing proven interventions like statins early enough to enough individuals, we can prevent 90% of CVD.
Step 1: Make statins OTC
Recently Alex Kesin presented a series of powerful arguments as to why statins should be available OTC (over-the-counter).
Statins are extremely safe - many drugs that are already OTC cause serious adverse events at 10-100x higher rates:4
There are also OTC drugs that are unsafe to take long term, most notably OTC “sleep aids” (Benadryl and Unisom).5 We also shouldn’t forget the litany of unsafe substances that are freely available “OTC” — things like cigarettes, vapes, alcohol, marijuana, and soda pop.
Statins are extremely safe, extremely effective, and extremely cheap. Alex Kesin estimated that the cost per quality-adjusted life year saved is “less than a fast food meal.” In 2018, Sniderman et al. estimated that for every seven people who take statins for thirty years, one life is saved.
The benefit of statins accrues at least linearly, and perhaps even super-linearly, with every year they circulate in the bloodstream.
Only 35% of people currently eligible under current guidelines are taking statins, according to research published in Annals of Internal Medicine.
The FDA has rejected applications to make statins OTC four times previously. None of those denials had anything to do with the safety or efficacy of the drug. Instead, the FDA feared that people would not be smart enough to know if they might benefit from taking a statin.
The FDA's assumptions about consumer intelligence and behavior have no basis in science, yet they've held those positions firmly.
One path forward here might be to meet the FDA “in the middle” on the issue. We could require pharmacies to administer a short survey to people before selling them statins OTC, similar to what is done with vaccines or pseudoephedrine. Indeed, a survey is exactly the sort of thing FDA now invites under their new "additional condition for nonprescription use" rule.
A 2021 study showed that when people took a survey on a simple smartphone app there was 96% agreement between the app’s output and the judgement of a physician for rosuvastatin. People used the app at home, and 20% of the people in the study had “limited literacy.”
Making statins OTC will help, but it would be a mistake to think that’s all we need to do.
Step 2: Combat statin hesitancy
There is a widespread belief that statins are likely to cause muscle pain, and surveys show that 10-20% of people report experiencing muscle pain while on them.
However, in the original clinical trials on statins, less than 5% of people reported muscle pain as a side effect. What’s going on here?
Two very interesting studies investigated statin use and muscle pain using a “within-subject” study design. In these studies, each participant was randomly alternated each month between taking a statin, taking a placebo, or taking neither. The first study had 60 participants and was published in two journal articles in 2020 and 2021. The second study had 200 participants and was published in 2021. Remarkably, both studies reached the exact same conclusion -- about 90% of “statin-induced muscle pain” is due to the nocebo effect.
What this means is that people report muscle pain while taking statins because they have a high expectation of experiencing it. This is consistent with a growing literature on predictive processing and psychosomatic pain, a topic I’ve touched on before.
Now, one thing that is important to mention about the two aforementioned studies is that they preselected people on statins who had complained about muscle pain to their doctor. If we look at some recent clinical trials, the side effect rate for muscle soreness is often around 10% in the treatment arm and 5% in the placebo arm, suggesting lower 50% nocebo effect. Altogether, the results suggest that statins actually do cause slight muscle pain in a small fraction of individuals (1-5%), but rarely ever cause more serious pain, except through the nocebo effect.
According to Matthews et al. (2016), the widespread belief that statins cause muscle pain can be at least partially traced back to a 2013 article published in the British Medical Journal. Unfortunately, the article mistakenly stated that muscle pain occurs in 18-22% of people. Six months later an errata was issued correcting the mistake, but the damage had already been done, as many fear-mongering articles had appeared in the media as a result (reporting on erratas, alas, does not sell newspapers).
Few things grab more clicks online than fear-mongering about a widely prescribed medication. Researchers have detected transient decreases in statin prescriptions following fear-mongering events in the media.
Step 3: Deploy gene editing
For one thing, it’s not clear how many people would actually start taking a statin regularly if they became OTC. In 2004 a statin called simvastatin became OTC in the UK, under the brand name Heart-Pro. By most accounts the uptake was quite low, and the manufacturer stopped selling it OTC in 2010. There were multiple reasons it was a dud. It had a high cost of about $400 annually, could only be shelved behind-the-counter, and could only be sold after a screening survey was administered by a pharmacist. Alex Kesin suggests that “faint-hearted marketing” might also have contributed to the lackluster uptake.
Even with cheap OTC that were marketed aggressively, it’s not clear if many at-risk people would rush out to start taking one.
Furthermore, the statistics on long-term adherence to statin therapy are not great, with adherence rates at one year around 40-61% across different populations. It’s hard to get people to stick to taking any drug consistently, especially drugs that aren’t treating an active condition. The worst adherence rates are found among the elderly and disabled, for whom making regular trips to the pharmacy can be a challenge.
If only there were a treatment that could be administered just once but would confer a lifetime’s worth of protection…
That’s where gene therapies come in.
There are a couple specific genetic mutations which greatly reduce CVD risk. A single mutation in the PCSK9 gene, which regulates low-density lipoprotein, confers a 50-88% lower risk of CVD with no known deleterious effects. Similarly, there are loss-of-function mutations in APOC3 or ANGPLT3 that result in 40-50% lower risk. These mutations are currently quite rare, with prevalences between 0.5 - 1%.
A company called Verve Therapeutics has been developing a gene therapy that edits the PCSK9 gene. They were recently acquired by Eli Lilly for $1.3 billion. The nice thing about targeting PCSK9 is you only have to edit the genes in the liver. The liver is one of the easiest tissue types to target in the body since all blood circulates through the liver’s dense network of capillaries.
Verve Therapeutics’ treatment has showed promising results in their Phase I trial and the company will be initiating Phase II trials later in 2025.
Step 4: Screen and intervene much earlier
Autopsy studies on teenagers show that about 10-15% have fatty streaks in their arteries. Fatty streaks are the earliest phase of atherosclerosis. Over the course of decades, fatty streaks turn into fatty plaques, which turn into fibrous plaques, which can then turn into blockages or calcified plaques.
The fact that atherosclerosis often starts in childhood has been known for a long time — the following figure is from a paper published in 1958:
Back then, autopsies showed that nearly all children had fatty plaque streaks, and many young adults had fibrous plaques. During the Korean war (1950 - 1953), 77% of soldiers who died (average age 22 years) were characterized as having “advanced atherosclerosis”. A lot of those GIs were probably smoking.
Today the situation has improved, due to a large decline in smoking, dietary changes, and other factors.
A large autopsy study conducted more recently found that more than half of people aged 15-37 had fatty streaks but only 10-20% had fibrotic plaques.
Why do some people have fibrotic plaques or even calcifications in their 20s while others have nothing? The fact is that it’s very hard to disentangle all the causative factors - it’s a complex mix of genetics, diet, and other exposures.
The good news is we don’t have to know exactly why someone is developing atherosclerosis at an accelerated rate to be able to intervene to slow or even stop its progression.
As early as 1961, doctors had recognized that high-risk individuals must be caught as early as possible, ideally during childhood years:
“I do think that pediatricians have definite responsibilities if we are ever to make progress in stopping the ravages of this disease.” - Russell L. Holman, M.D., 1961.
Today, high risk individuals are found using either the Framingham Risk Score or the Pooled Cohort Equations risk score. Individuals who score high may be offered statins. For those with moderate risk, additional testing is often run, like a coronary artery calcification (CAC) scan or blood testing for C-reactive protein.
This is all well and good, but there are several other types of screening we should be doing a lot more of:
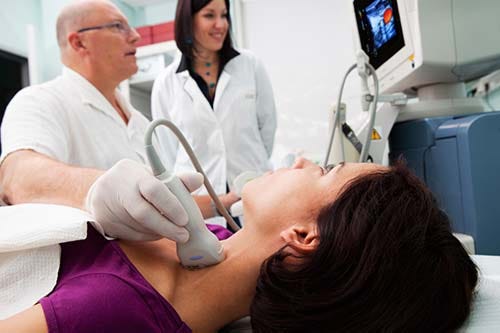
The first is a simple ultrasound measurement of the thickness of the carotid artery wall. The test only takes about ten minutes to complete.
You might be wondering about CT and MRI scans. Screening using a CT scan (like a CAC scan of the heart) is a bit controversial due to the non-negligible radiation exposure, but (assuming the CT is done correctly), the risk/reward is generally favorable. MRI could in theory be used for screening, but it’s currently way too expensive.
However, we can utilize existing medical images. For instance, small vascular calcifications are often observed on mammograms:
Atherosclerotic plaques in the heart, aorta, and major arteries can be observed in most non-contrast CT scans and in some MRI scans.6 Plaques in the aorta can be observed on DEXA scans, which are typically only used to measure bone mineral density.
If calcifications or plaques are present on a scan, they might be noticed. If they are noticed, they might be mentioned in the radiology report. If that happens, then the report might be read in full by a doctor. After that, the doctor might tell the patient they have plaques. Finally, the patient might act on that information. You’ll notice there were a lot of “mights” there.
In actuality the doctor is very unlikely to tell the patient about the calcifications or plaques unless they are deemed an imminent threat, due to a widespread dislike in the medical establishment towards screening and “incidental findings.”7
That’s what needs to change.
To wrap up this section, I want to discuss genetic testing. There are a handful of genetic variants which dramatically increase CVD risk, but they are pretty rare. A dramatic example is familial hypercholesterolemia, which occurs in about 1 in 250 people. There is a strong case for testing for rare genetic diseases during IVF so parents can avoid creating children with such conditions.
CVD has been estimated to be 40-60% heritable. In theory, polygenic risk models should have a lot of utility. Currently, however, their ability to stratify individuals by risk is somewhat limited:
Thus, the incremental value of using a polygenic risk score on top of standard screening methods (like the pooled cohort equation) is low at the moment (see this paper).
Conclusion
“… most physicians and cardiology experts would still insist that one’s thirties are too young to begin to focus on primary prevention of cardiac disease…
… Once you understand that apoB particles—LDL, VLDL, Lp(a)—are causally linked to CVD, the game completely changes. The only way to stop the disease is to remove the cause, and the best time to do that is now.
Still struggling with this idea? Consider the following example. We know that smoking is causally linked to lung cancer. Should we tell someone to stop smoking only after their ten-year risk of lung cancer reaches a certain threshold? That is, do we think it’s okay for people to keep smoking until they are sixty-five and then quit? Or should we do everything we can to help young people, who have maybe just picked up the habit, quit altogether? When viewed this way, the answer is unambiguous. The sooner you cut the head off the snake, the lower the risk that it will bite you.” - Peter Attia
The path to reducing cardiovascular disease is clear. It starts by getting more people on statins earlier. For the rare patients who can’t tolerate statins, there are a number of other options.8 In the future, gene therapies will allow a one-shot treatment, ameliorating the issue of statin non-compliance.
When I said in the subtitle that “cardiovascular disease is a policy choice”, I was thinking not just about government policy but also healthcare policy writ large.
The astute reader may have noticed some gaps in the steps I outlined. I don’t know what the best ways are to combat misinformation about statins, and I don’t know the best ways to get doctors to screen earlier and prescribe statins earlier. If you have ideas, please leave them in the comments!
A little aside on terminology — “cardiovascular disease” is a bit of an umbrella term. It includes heart attacks, heart failure, strokes, aortic aneurysm, cardiomyopathy, and cerebrovascular disease. Sometimes Chronic Lower Respiratory Disease is also included. Other times, it is defined more narrowly as “myocardial infarction, cerebrovascular accident, or congestive heart failure” The interventions in this post target the cause for the vast majority of all heart disease, which is atherosclerosis. Some authors, like Peter Attia, prefer to discuss atherosclerotic cardiovascular disease (ACVD), but for simplicity I just refer to CVD.
One startup is working on a bold new therapy to reverse atherosclerosis - Repair Biotechnologies. It appears their approach involves using a targeted “suicide” gene therapy to kill senescent cells within plaques.
In a nutshell, the mechanism is that apoB particles (LDL, VLDL, Lp(a)) get stuck to the artery wall and become oxidized. This creates reactive oxygen species that damage the wall. Eventually, immune cells called monocytes (large white blood cells) come along and turn into macrophages. The macrophages try to eat the apoB particles and the cholesterol they carry inside. If they eat too much, they become foam cells, a type of sticky cell that becomes permanently stuck to/within the artery wall. The process then feeds on itself, creating a “fatty streak.” Eventually, the body attempts to contain and stabilize the accretion by covering it in fibrotic tissue. The final stage involves calcification of the plaque. The key is that apoB particles are the root cause, and statins reduce their density in the blood stream.
References: Orlistat: Douglas I., et al, The Lancet 2013, Acetaminophen: Goldberg et al. 2015, NSAIDs: Lanas A, et al. Am J Med 2005, Statins: Collins R et al. The Lancet 2016 .
Benadryl and Doxylamine cause long-term harm via their anticholinergic action, something I wrote about before. In that article I make the case that both drugs should probably not be OTC, and short-acting orexin antagonists probably should be.
When I worked at NIH between 2019-2021 I developed a system that measures plaque in the heart and aorta present in a non-contrast CT scan. We also showed that by adding measurements of visceral fat, muscle, and liver fat we could improve cardiovascular disease risk prediction slightly. Another approach I developed involves feeding an abdominal CT scan directly into an ML model which outputs cardiovascular risk. A startup called Nanox has an system that can automatically measure and visualize coronary plaques from a standard non-gated chest CT scan. In one hospital, 60% of the patients flagged by their system were previously unknown to have high cardiovascular risk.
There was once a startup called Corventive that was trying to improve this situation, and in 2023 I spoke with its founder. One of their products was a very simple “AI” system that detects if calcifications are mentioned in a patient’s mammography reports. If so, the idea was that Coreventive’s software would start bugging the doctor to order follow up tests (like blood tests or a coronary artery calcification scan). Such procedures are billable to insurance, so by using Coreventive’s system the hospital would make more money. In my view patients would also benefit from such a system assuming they are given a treatment like statins rather than being told to eat healthy and exercise (which is unlikely to do anything). In that case it would be a win-win. Unfortunately, hospitals don’t a lot of spare change lying around for new software, and deployment of software into hospital environments comes with a lot of challenges unless you’re able to work with one of the large EHR vendors. It looks like Corventive’s website went dark in April 2025.
In Outlive, Peter Attia says he prescribes drugs in this order - rosuvastatin (Crestor), bempedoic acid (Nexletol), ezetimibe (Zetia), a PCSK9 inhibitor.







Is there a "Cardiovascular disease is a personal choice" version? I've been trying hard recently and got my LDL to below 80 but it's been stubborn to go significantly below that (my most recent test result was 75 but it's really been a lot slower since reaching 80)
I'm having trouble understanding the paper that suggests a 90% (for men, 78% for women) decrease in risk. I think that reflects the difference between the highest and lowest "PDAY" scores? I don't quite figure out what that is, but it seems like most people might have intermediate scores, meaning the drop in risk is less?